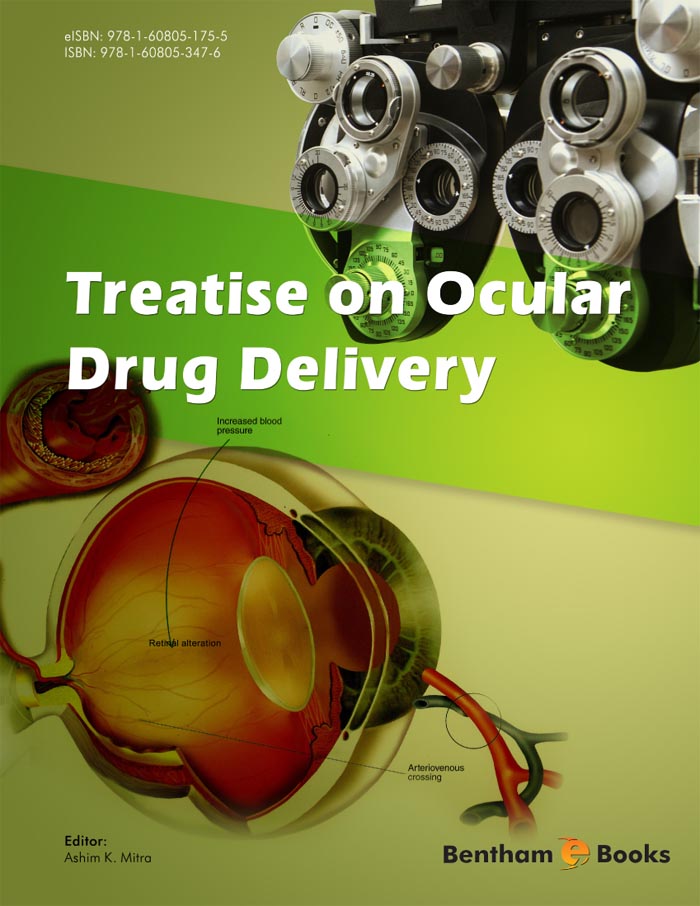
Therapeutic efficacy of pharmacotherapy in the eye necessitates delivering complex drug substances to sequestered active sites for the appropriate duration. Achieving this is fraught with challenges from issues of bioavailability to simple non-compliance, whether that’s due to physical limitation such as in the elderly, lifestyle factors or simply not self-administering medication. Drug delivery can greatly improve the therapeutic outcome in these cases. Moreover, in certain diseases drug delivery may not only enhance performance of an active, but is an absolute requite for function.
Despite new and novel therapies for posterior segment diseases there is still a very large unmet medical need to treat serious ocular conditions. Vision threatening back of the eye diseases such as wet age-related macular degeneration, diabetic macular edema, uveitis and geographic atrophy are leading causes of blindness. Various therapeutic classes of drugs are showing promise at a receptor or ligand level for affecting these diseases. Promising new drug substances range from antibodies, antibody fragments and peptides to aptamers and siRNAs. In the case of some drugs, such as siRNAs, the lack of an effective delivery system may prevent fulfilling the promise of an entire therapeutic class.
Unfortunately, delivering these drugs to their intended target; the choroid, RPE and vitreoretinal space remains illusory. Without new and novel drug delivery strategies, effective pharmacologic intervention in these diseases may never come to fruition. Drug delivery to the eye is multifactorial and poses significant challenges. Bioavailability to the anterior segment of the eye remains low from topical administration despite decades of research. In fact, the challenges to ocular drug delivery are becoming exponentially greater with therapies directed towards posterior segment diseases and a much broader palette of drug substances to be delivered. The present eBook not only covers these issues in significant detail, but the leading experts in the field authored the chapters. The present eBook offers an integrated understanding of anatomy and physiology, disease state, drug absorption and disposition and drug delivery.
The first five chapters of the eBook lay the foundation for ocular drug delivery and the current state of the art. Chapter 1 nicely deals with the anatomic and physiologic constraints to drug delivery. Chapter 2 explores delivery beyond conventional topical and systemic approaches. Taking advantage of non-conventional routes of delivery minimizes some of the issues with conventional topical therapy including tear dilution, rapid precorneal drainage and may also buffer systemic absorption to some extent. Additionally, these novel routes offer new and novel opportunities for sustained and controlled delivery to the eye. This chapter differentiates conventional and novel routes and discusses their relative merits. Chapters 3 and 4 deal with anterior and posterior segment drug delivery, respectively. The various attempts to improve anterior segment drug delivery are described. Static and dynamic barriers to drug delivery are discussed. A decade ago little consideration was given to the dynamic barriers to penetration as well as the role of enzymes and transporters in posterior segment drug delivery. The field is just now realizing the impact of these factors and Chapter 4 describes these critical and newly defined variables.
Specific strategies for achieving effective drug delivery to the eye are detailed in Chapter 5 through 7 and Chapter 10. In Chapter 5, biodegradable polymers for drug delivery are reviewed. This class of polymers represents a significant component of many posterior segment drug delivery strategies. Unfortunately, a detailed understanding as it relates to the eye is often missing from the ocular formulator’s arsenal. This is a crucial chapter for anyone interested in ocular drug delivery. Drug delivery in a broader context is dealt with in chapter 6 and includes erodible and non-erodible systems. Implants, microspheres, liposomes and semi-solids as well as more complex delivery systems are discussed. These strategies have been shown to be effective in circumventing many of the barriers to ocular absorption, but none are without potential side effects. Chapter 7 addresses enhancing the permeation of these barriers, rather than circumvention, through techniques such as ultrasound, microneedles and prodrug modification.
The next two chapters cover critical areas for the field. The eye is not readily amenable to serial sampling and as such it is difficult to reasonably power ocular pharmacokinetic studies. Microdialysis as a tool to address the limitations of classic ocular pharmacokinetic techniques is discussed in Chapter 8. This is a tool that will go a long way in facilitating the development of effective treatments for ocular diseases. However, getting new delivery systems approved and protected is as important as innovating new technologies. Unmet patient needs are not addressed until a drug delivery system is shown to be safe and effective in well-controlled clinical trials, approved through the relevant regulatory bodies and commercialized for use. Chapter 9 discusses US regulatory requirements and guidelines relevant to ophthalmics as well as recent patents in the field. The final chapter of the eBook, Chapter 10, discusses the exiting new area of nanotechnology in drug delivery. Nanotechnology advances such as liposomes, niosomes, nanoparticles and dendrimers among others are fully explored
The present eBook covers in significant detail the issues and resolutions with developing drug delivery systems for the eye. Moreover, key researchers in the field authored this eBook. This eBook serves as a great reference for anyone involved in treating ocular disease including: ophthalmologists and other clinicians, pharmaceutical formulation scientists, ocular pharmacokineticist and pharmacists amongst others. This reference should be in the armamentarium of any scientist serious about drug delivery to the ocular tissues.
Patrick M. Hughes
Formulations and Drug Delivery Sciences,
Allergan, Inc.