Alexander D. Pogrebnjak1 and Vyacheslav M. Beresnev2
1Sumy State University, Sumy Institute for Surface Modification, R.-Korsakov Str., 2, 40007 Sumy, Ukraine;
E-mail: alexp@i.ua and 2Kharkov National University V.M. Karazina, sq. Svobody, 4, 61022 Kharkov,
Ukraine; E-mail:Beresnev-scpt@yandex.ru
In recent years, a research of materials, which are composed of submicron nanosized grains and clusters,
are swiftly developing due to already existing and/or potential applications in many technological fields
such as electronics, catalysis, magnetic data storage, structure components, etc.
Metallic and ceramic materials with a submicron and nanocrystalline grain structure are now widely used as
construction elements and functional layers in a modern microelectronics, as sites of devices in aviation and
space engineering, and as hard wear resistant coatings in industry. To satisfy the technological requirements
of these industrial fields, the size of structure elements is to be decreased to a submicron and a nanometer
range. However, when a size of structure element decreases to a nanometer range, a material starts to
demonstrate radically new physical and mechanical properties in comparison with a bulky base. Researches
of these nanosized structures (nanostructures) rank among nanotechnological directions. Development and
researches of nanostructured materials (further referred to as nanomaterials) and nanostructure properties
obtained under various conditions are very important components of these scientific-technological
directions. A material, a structure of which is composed of grains of about 0.3 to 0.04 μm size, is
considered as a submicrocrystalline [1-3]. A material of smaller grain size is considered as a nanomaterial.
A nanomaterial (a nanocrystal, a nanocomposite, a material with a nanophase structure, etc.) is to be
understood as a material, in which structure elements (a grain, a crystallite, a fiber, a layer, a pore) do not
exceed a limit of 100 nm (1 nm = 10-9 m), at least, along one crystallographic direction. According to size
of a structural unit, the nanomaterial is conventionally subdivided into a nanocluster and nanocrystalline
material. A nanocluster material is subdivided into small (3 to 12 number of atoms, 100% of surface atoms,
without an inside layer), big (13 to 150 number of atoms, 92 to 63% of surface atoms, including 1 to 3 inner
layers), and giant nanocluster material (151 to 22000 number of atoms, 63 to 15% of surface atoms,
including 4 to 18 inside layers). Conventionally, a cluster top boundary corresponds to such amount of
atoms that an addition of one more atom already cannot change physical-chemical properties of this cluster.
Theoretical calculations, which were confirmed by experimental researches for a cluster containing not less
than 300 atoms, demonstrated that an icosahedrons structure is the most stable one. When an amount of
cluster atoms increased, an elastic deformation energy quickly rouse in a proportion to their volume, and
consequently, this icosahedrons structure is destabilized forming a face-centered cubic lattice [4].
A structure unit with a higher amount of atoms and 3 to 40 nm grain size ranks among a nanocrystal. This
nanocrystalline material has various forms and demonstrates unique chemical, physical, and mechanical
properties. A grain size is limited by the maximum size of the nanostructure elements and depends on some
critical parameters (a size effect): a free range length of carriers participating in an energy transfer, a size of a
domain/a domain wall, a diameter of a Frank-Reed loop, a de Broglie wave length, etc. This size effect sharply
changes quality and properties of the nanostructured system and indicates a special condensed material state,
which exists only in the nanostructured material. Today, the nanostructured material can be formed on the basis
of various metals and alloys, and with the help of specially developed technological methods.
In recent years, a definite progress had been achieved in physical researches and technologies of the
nanostructured material fabrication. In particular, an important stage of these researches is a systematic
study of microprocesses occurring in a phase interface in the course of nanostructured system formation.
This systematic study stimulated an appearance of calculation methods, which are employed to predict
optimal technological parameters and promising ways of the nanostructured material formation.
A whole number of publications, monographs, and papers [5-11] report about technologies, structures,
properties, and applications of the nanomaterial and the nanostructure.
Here, we present only a description of individual representatives and classes and do not reflect, to a full
extent, features of this modern direction. Why is there this modern interest in a nanotechnology, in general,
and in a nanostructure study, in particular?
On one hand, nanotechnologies allow formation of a principally new material, which can find its application in
future, since it is compact and functionable. It plays an important role in the formation of principally new
elements for future nanodevices, which are dependent on physical principles employed for their functioning.
On the other hand, the nanotechnology is an extremely wide interdisciplinary direction, uniting specialists
working in a field of physics, chemistry, materials science, biology, technology, directions of
intellectual/self-organized systems, high-technological computer engineering, etc. Finally, solving
problems arising in the field of nanotechnologies, and, first of all, in the process of researches, scientists
find many gaps existing both in fundamental and technological knowledge. All above mentioned excites a
concentrated interest of a scientific and engineering society to this direction [12-21].
In many technologically advanced countries such as USA, United Kingdom, Japan, China, Russia, national
programs, which are specified at an intensive development of various directions of the nanotechnology and
formation of new nanostructures, are accepted and have started to be actively introduced into a practice.
Now, several basic types of the nanomaterials are known [1, 4].
VARIETY OF NANOMATERIALS
A nanomaterial has a number of structure characteristic features, which are the parameters relating to a
structure as a whole and those identifying its individual elements. In their turn, the structure characteristic
features of the nanomaterials are reflected in an unusual display of their properties. Since the nanomaterial
is a basic unit of a nanosystem, properties of the nanosystem to a considerable degree depend on the
nanomaterial properties.
Variety of nanomaterials is immense and every type is characterized by a specific structure and, as a
consequence, specific properties. The characteristic features of the nanomaterial and the system formed on
its basis, first of all are manifested in a size effect, among which a quantum effect takes a special place.
According to degree of their structure complexity, the variety of nanomaterials is subdivided into materials
composed of individual nanoparticles and those composed of nanostructures (Fig. 1).
A nanoparticle is a nanosized complex of atoms and molecules, which are interrelated in a definite way.
Figure 1:

Figure 1: A classification of nanomaterials according to their structure characteristic features.
The following types of the nanoparticles are identified:
-
Nanocluster, which is sorted as an ordered cluster characterized by a definite order in an
arrangement of atoms and molecules and a strong chemical bond and a non-ordered
nanocluster characterized by a disordered arrangement of atoms and molecules and a weak
chemical bond;
-
Nanocrystal (a crystalline nanoparticle), characterized by the ordered arrangement of atoms
and molecules and the strong chemical bond like a bulky crystal (a macrocrystal);
-
Fullerene, which is composed of carbon atoms (or atoms of another element) forming a
structure looking like a spherical carcass;
-
Nanotube, which is composed of carbon atoms (or atoms of another element) forming a
structure looking like a cylindrical carcass closed at its both ends;
-
Supermolecule, which is composed of “a host molecule” with a three-dimensional structure,
in a cavity of which a “guest molecule” is arranged;
-
Biomolecule, which is a complicated molecule of biological origin characterized by a
polymer structure(DNA, a protein);
-
Micelle, which is composed of molecules of a surface-active matter forming a sphere-like
structure;
-
Liposome, which are composed of molecules of a special organic compound like a
phospholipid forming a spherical structure;
A nanostructured material is an ensemble of nanoparticles. Nanoparticles play a role of a structure element
in such material. A type of the nanostructured material depends on a character of interrelation existing
between nanoparticles: a consolidated material and a nanodispersed one.
The consolidated material is a compact solid-phase material, which is composed of nanoparticles with a
fixed spatial position in the material volume and rigidly-directly bound to another one.
The consolidated material is:
-
Nanocrystalline material, which is composed of nanocrystals usually called a nanograin or a
nanocrystallite;
-
Fullerite, which is composed of fullerenes;
-
Photon crystal, which is composed of ordered-in-space elements, a size of which is
comparable with a half-length of a photon wave in one, two or three directions;
-
Layered composite material (with a superlattice), which is composed of various material
layers of a nanosize thickness;
-
Matrix component, which is composed of a solid base (a matrix), in the volume of which
nanoparticles (nanowires) are distributed;
-
Nanoporous material, which is characterized by the presence of nanopores;
-
Nanoaerogel, which is composed of an interlayer of a nanosize thickness separating pores.
A nanodispersed material is a dispersed system with a nanosized dispersion phase.
In addition to the above mentioned matrix nanocomposite materials and nanoporous materials, the
nanodispersed materials cover:
-
Nanopowder, which is composed of contacting other nanoparticles;
-
Nanosuspension, which is composed of nanoparticles free-distributed in the liquid volume;
-
Nanoemulsion, which is composed of nanodrops of a liquid free-distributed in a volume of
another liquid;
-
Nanoaerosol, which is composed of nanoparticles and nanodrops free-distributed in a volume
of a gaseous medium.
Specimens of various nanostructured materials are often bulky, i.e. are characterized by a micro-and macrosize,
whereas their structure elements are nanosized.
Effects, which are related to the small size of composing structures, may manifest themselves in a different
way in various nanomaterials.
For example, a specific surface of the nanocrystalline and nanoporous material is crucially larger, i.e. a
fraction of atoms arranged in a thin (about 1nm) near-surface layer radically arises. This increases the
reaction ability of the nanocrystal, since atoms, which are arranged in the surface, have unsaturated bonds
in contrast to atoms, which are arranged in the material bulk, since they are bound with surrounding atoms.
A change in atomic ratio between the surface and the bulk atoms may result in atomic reconstruction, in
particular, in a change of an atomic arrangement order, an interatomic distance, and a crystalline lattice
period. The size dependence of a nanocrystalline surface energy predetermines a corresponding dependence
of a melting temperature, which is lower for the nanocrystal than for the macrocrystal. As a whole, heat
properties of the nanocrystal are crucially different, which is related to a character of atomic heat
oscillations.
When the size of ferromagnetic particle decreases to a certain critical value, the domain separation becomes
energetically disadvantageous. As a result, polidomain nanoparticles become single domain and acquire
special magnetic properties, which are manifested in supermagnetism.
The fullerene and the nanotube are characterized by very unusual properties due to their specific structure.
This is true also for the molecular and biomolecular complex functioning according to laws of molecular
chemistry and biology.
Peculiarities of a structure and properties of an individual nanoparticle affect in a definite way a structure
and properties of the consolidated materials and the nanodispersion, which are formed on their basis.
A typical example is a nanocrystalline material, which is characterized by a decreased grain fraction, and,
respectively, an increased fraction of interfaces occurring in the material volume. Simultaneously, a change
of structure characteristics of both grains and interfaces takes place. As a result, mechanical properties of
the nanocrystalline material significantly change. The material demonstrates a superhardness of a
superplasticity under definite conditions.
Electron properties of the nanostructure, which are conditioned by quantum effects, are of a special interest
for practical applications.
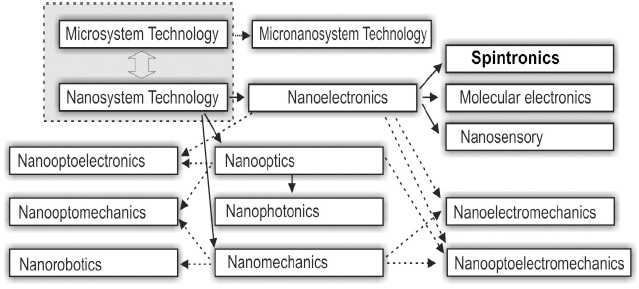
Figure 2: A classification of types of nanosystem-based devices according to their functional purposes.
The nanomaterial serves as a basis for the development of nanosystems with various functional purposes,
which in their turn are subdivided into an electron, an optical, and a mechanical nanosystem, according to
the principle of functioning, Fig. 2. An action of the electron nanosystem is based on transformation of an
electrical signal, that of the optical one-on transformation of an optical signal (light) into the electrical one
and vice versa, and the mechanical nanosystem transforms a mechanical motion.
Sets of definite characteristics of nanosystems are employed in definite fields of engineering such as
nanoelectronics, nanooptics, and nanomechanics. Development of various types of nanosystems is closely
interconnected and results in fabrication of more constructively complicated and integrated nanosystems,
such as nanooptical-electron, nanoelectrical-mechanical, nano optical-mechanical, and nano optical-electromechanical
systems.
The development of nanosystems is undoubtedly a new step, which will enable a future progress of
microsystems. In practice, nanosystems are built-in various Microsystems forming in this way a promising
direction of a modern system units (devices) such as a micronanosystem equipment.
A consolidated material-is a compact, a film, or a coating formed from a metal, an alloy, or a compound using a
powder technology, an intensive plastic deformation, a controlled crystallization from an amorphous state, and
various other techniques, which are currently applied for deposition of a film and a coating.
A nanosemiconductor, a nanopolymere, and a nanobiomaterial may exist in an isolated or a partially mixed
(consolidated) state.
Fullerene and nanotube became an object of researches since the moment, when Sir Harold (Harry) Walter
Kroto (1985) found a new carbon allotrope form-a cluster C60 and C70, which was called fullerene. This
new carbon form attracted much more attention, when the carbon nanotube was revealed in a graphite
product after an electrical-arc evaporation (Sumio Iijima, 1991).
Nanoparticles and nanopowder represent a quasizero grain size structure having various compositions and
a size of which usually does not exceed a nanotechnological limit. A difference is that the nanoparticle is
isolated, while the nanopowder is an aggregate. In a similar way, the nanoporous material is characterized
by a pore size, which, as a rule, is not less than 100 nm.
A supermolecular structure is a structure, which is formed as a result of so-called non-covalent synthesis
accompanied by formation of weak bonds (a Van der Waals, a hydrogen type, etc.) between molecules and
their ensembles.
The nanomaterial is not a “universal” material; it is a vast class of many various materials joining different
families. In addition, there exists a delusion that the nanomaterial is a material composed of very small “nano”-particles. In reality, many nanomaterials are not composed of individual particles; they are
complicated micro objects nanostructured either in a surface or in a volume. Such nanomaterials are
considered as a special state of matter, since properties of these materials, which are composed of
nanostructured and nanosized elements, are not identical to properties of a volume material.
So, the nanomaterial is characterized by several basic features, which position it beyond any competition in
comparison with other matters.
First, the nanomaterial is composed of very small objects, which cannot be seen with a naked eye. It
represents a “super miniaturization”, which leads to a possibility that more and more quantity of functional
nanodevices can be placed at an area unit. This is vitally important, say, for nanoelectronics or very dense
magnetic information recording, which can reach 10 Tirrabit per a square centimeter.
Second, the nanomaterial has a large surface area, which promotes a hastened interaction inside and within
a medium, to which it is placed in. For example, a catalytically active material can hasten a chemical and
biochemical reaction by a factor of ten, thousand, and even a million [22-30].
Decomposition of water into hydrogen and oxygen for needs of a hydrogen power engineering, which is
realized in the presence of titanium dioxide nanoparticles (everyone knows it as a component of titanium white
paint), seems to be very interesting. A nanofilter can screen bacteria or efficiently absorb impurities and toxins.
Third, the nanomaterial has unique physical and mechanical properties, and this means that such a matter is
in a specific “nanosized” state. Changes found in the nanomaterial fundamental characteristics are
conditioned not only by a small grain size, but also by a quantum-mechanical effect, in which an interface
plays a dominating role. The effect arises when the grain size is so “critical” that is commensurable to a socalled
correlation radius or other physical parameters (for example, a free electron and a phonon range, a
coherence length in superconductors, a magnetic domain or a nucleon size of solid phase, etc.). This makes,
in particular, a semiconducting material to be an ideal element for a perfect energy-consuming laser and
light emission. Hardness of an individual carbon nanotube exceeds that of the best steel by a factor of ten.
At the same time, it has many-fold advantage in a specific mass. All above-mentioned characteristics fully
explain the fact that even a gram of the nanomaterial may be much more efficient than a ton of an ordinary
matter, and that its industrial production is not a problem of quantity, ton, and kilometer, but that of a
human thought quality, i.e. “know-how”.
Nanotechnology is an extremely complicated, professional, interdisciplinary field, which needs joined
efforts of chemists, physicists, specialists in material science, mathematicians, medics, specialists in
calculation methods, etc. Deep scientific fundamentals are admirably interwoven in a field of nanomaterials
with aspects of a human knowledge and practical applications.
In this eBook, we report fundamental data concerning structure, properties, and application of the modern
nanomaterials. In the First Chapter, we present general information about nanomaterials, their structure
features, size effect on structure formation and on physical-mechanical properties.
In Chapters 2 and 3, we present information about structure and properties of a nanoporous and an
amorphous material.
In Chapter 4, we consider certain properties of fullerene and nanotube. Chapter 5 deals with a
nanocomposite based on a polymer. Chapter 6 is devoted to methods, which are currently employed for the
nanomaterial fabrication, since these new methods really gave rise to a violent development of this field.
Physical research methods, namely, novel methods employed for surface studies are presented in Chapter 7.
In Chapter 8, we consider mechanical and thermal properties of a nanocrystalline film and a nanocomposite
coating, which are fabricated using physical deposition methods.
Chapter 9 is devoted to an application of the nanocrystalline material, which is employed in an engineering
society.
REFERENCES
[1] Morokhov ID, Trusov LI, Chizhij SP. Unltradipersion Metallic Media. M Atomizdat 1977.
[2] Gleiter H. Nanostructured Materials. Basic Concepts and Microstructure. Acta Materialia 2000; 48: 1-29.
[3] Seigel RW. Nanostructured Materials-Mind over Matter nanostruct. Mater 1993; 3: 1-18.
[4] Larikov LN. Nanocrystalline Compounds of Metals Metallo-Fizika I Noveishie Tekhnologii 1995; 17: 56-68.
[5] Andrievskii RA, Ragulia AV. Nanostructured Materials. Moscow: Academia 2005.
[6] Roko MK, Wiliyams PS, Alivisatos P. Nanotechnologies in the Next Ten years. Prediction of Researching
Directions; Translation. Ed. Andrievskii RA. Moscow: Mir 2002.
[7] Liakishev NP, Alymov MI. Nanomaterials for Structural Materials. Nanotechnologies in Russia 2006; 1: 71-81.
[8] Gusev AI. Nanomaterials, Nanostructures, Nanotechnologies. Moscow: Fizmatlit 2005.
[9] Gusev AI, Rempel AA. Nanocrystalline materials. Moscow: Fizmatlit 2000.
[10] Pozdniakov VA. Physical Material Science for Nanostructured Materials. Moscow: MGIU 2007.
[11] Ragulia AV, Skorokhod VV. Consolidated Nanostructured Materials. Kiev: Naukova Dumka 2007.
[12] Sergeev TB. Nanochemistry Moscow: MGU 2003.
[13] Andrievskii RA, Glezer AM. Size Effects in Nanocrystalline materials. I. Features of Structure. Thermal-Dynamics.
Phase Equilibrium. Kinetic Phenomena. The Physics of Metals and Metallography 1999; 88: 50-73.
[14] Andrievskii RA, Glezer AM. Size Effects in Nanocrystalline Materials. II. Mechanical and Physical Properties. The
Physics of Metals and Metallography 2000; 89: 91-112.
[15] Pogrebnjak AD, Shpak AP, Azarenkov NA, Beresnev VM. Structure and Properties of Hard and Superhard
Nanocomposite Coatings. Physics Uspekhi. 2009; 52(1): 29-54.
[16] Noskova NI, Muliulukova RR. Submicrocrystalline and Nanocrystalline Metals and Alloys. Ekaterinburg: Ural’skoe
Otdelenie RAN 2003.
[17] Suzdalev IP. Nanotechnology: Physical Chemistry of Nanoclusters, Nanostructures, and Nanomaterials. Moscow:
KomKniga 2006.
[18] Valiev RZ, Aleksandrov IV. Nanostructured Materials, Fabricated by Intensive Plastic Deformation. Moscow: Logos
2000.
[19] Andrievskii RA. Nanomaterials: Concepts and Modern Problems. Rus Chem J 2002; XLVI: 50-56.
[20] Glezer AM. Amorphous and Nanocrystalline Structures: Similiarities, Difference, Mutual Transitions. Rus Chem J
2002; XLVI: 57-63.
[21] Andrievskii RA. Thermal Stability of Nanomaterials. Russ Chem Rev 2002; 71: 967-981.
[22] Demikhovskii VJ, Vugalter GA. Physics of Quantum Small Grain Size Structures. Moscow: Logos 2000.
[23] Harris P. Carbon Nanotubes and Related Structures. New Materials of XXI Century. Translated by
L.A.Chenazatonskii. Moscow: Tekhnosfera 2003.
[24] Pomogailo AD, Pozenberg AS, Ufliand IE. Nanoparticles of Metals in Polymers. Moscow: Khimiia 2000.
[25] Shevchenko SV, Stetsenko NN. Nanostructured States in Metals, Alloys, and Intermetalloid Compounds: Methods of
Fabrication, Structure, Properties.Progress in Physics of Metals 2004; 5: 219-255.
[26] Shik AJ, Bakuleva LG, Musikhin SR, Rozhkov SA. Physics of Small Grain Size Systems St.-Peterburg: Nauka 2001.
[27] White Book on Nanotechnologies: Researches in the Field of Nanoparticles, nanostructures, and Nanocomposites in
Russian Federation (Materials of the First All-Russia Meeting of Scientists, Engineers, andfabricationrs in the Field
of Nanotechnologies) Moscow: LKI 2008.
[28] Pul Ch, Ouens F. Nanotechnologies. Translation by Moscow: Tekhnosfera 2004.
[29] Golovin YuI. Introduction in nanotechnology Moscow: Mashinostroenie 2003.
[30] Shorshorov MKh. Ultradispersion Structure State of metallic Alloys. Moscow: Nauka 2001.



